Subject: FDA
PeekMed® Unveils New Automated Orthopedic Solution for the US
BRAGA, Portugal, Feb. 21, 2023 /PRNewswire/ -- PeekMed® sets new expectations for the orthopedic technology market with the launch of a web-based automated planning solution. With this 510(k) clearance, from the U.S. Food and Drug Administration (FDA), this new advanced AI solution represents a new era for orthopedic technology, and for the whole value chain in orthopedic surgery.
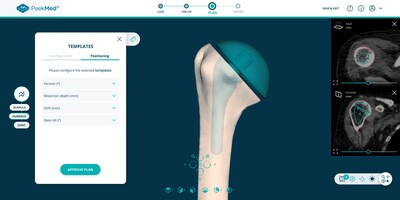
Powered by artificial intelligence and deep learning technologies, the new PeekMed® solution, now web-based, makes everything faster and simpler for orthopedic surgeons. This new product supports live automatic segmentation of CT scans and X-Rays, automatic landmarks detection, and automatic planning. Through high-performing technologies, an accurate plan is presented to the surgeon, live, in under 30 seconds. All planning steps are automatic, and the surgeon only has to approve them (or perform changes if needed).
The creation of this new technology resulted from the need to solve the challenges of orthopedic surgeons and other players whom PeekMed® works with: to increase efficiency and reduce time-consuming tasks from the surgery planning process.
João Pedro Ribeiro, Chairman & CEO of PeekMed®, explains that "With this FDA clearance, PeekMed® is ready, once again, to be a game-changer in the integration of technology with orthopedics. Having the ability to get a plan in less than 30 seconds is just incredible and a huge achievement for our team. We understood the pains of our surgeons, who were waiting hours, or sometimes days, to get a case planned. We aim to solve those challenges!"
With experience in the development of technological solutions for orthopedics, PeekMed® is already a trusted partner of more than 3500 orthopedic surgeons worldwide.
"We believe American orthopedic professionals will trust our new product and its development process. PeekMed® is very rigorous with itself because, as a medical device company, patient safety is of utmost importance and a central matter for us. PeekMed® is now one step closer to revolutionizing the orthopedics field!" he finishes.
Despite these automatic processes, there are no new severe risks since the generated 3D models, landmarks, and the planning are always reviewed and validated by the surgeons and when needed, they can be manually adjusted. Their goal is to make the surgeon's life easier, reduce the probability of error and save time and money.
PeekMed® web is capable of performing a total overview of the surgery by representing medical images in a 2D or 3D environment, performing relevant measurements on those images, and adding templates. All PeekMed® previous features have been maintained but with a new, faster, and more intuitive interface, that easily connects with other orthopedic solutions in the most used anatomical regions of the musculoskeletal system (Knee, Hip, and Upper Limb) of adults.
PeekMed® 's reentrance into the US market coincides with its presence at the American Academy of Orthopaedic Surgeons Annual Meeting (AAOS) in Las Vegas. Here it will be possible to experience PeekMed® web and much more!
Photo - https://mma.prnewswire.com/media/2005352/PeekMed_Web.jpg
SOURCE PeekMed
These press releases may also interest you
|
News published on and distributed by: