Subjects: WOM, RCL
Mason Vitamins Inc. Voluntary Recalls Healthy Sense Daily Multiple with Iron tablets and People's Choice Women's Daily Vitamins with Iron tablets sold in the U.S due to inconsistent product labeling with the product
MIAMI LAKES, Fla., Nov. 18, 2022 /PRNewswire/ -- Mason Vitamins Inc. has issued a nationwide voluntary recall of the specific lot of Healthy Sense Daily Multiple with Iron and People's Choice Women's Daily Vitamins with Iron due to Vitamin A, Vitamin B12, Vitamin C, Vitamin E and Pantothenic Acid amounts being lower than the declared amount on the label which was determined during an FDA inspection.
The products were sold nationwide at Bargain Barn, 99 Cents Only, Fruth Pharmacy, Joe V's Smart Shop, Rose's Discount Stores, Rex Discount Pharmacy, Star Discount Pharmacy, Propst Discount Pharmacy, Dollar Tree and limited distributors.
Product Name | Size | UPC Code | Lot/Expiration Date |
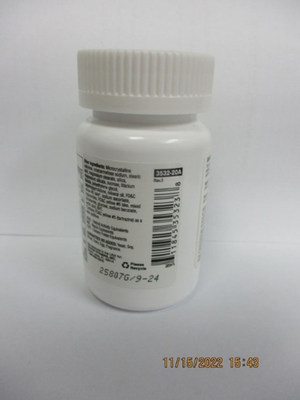
Healthy Sense Daily Multiple with Iron | 20 tablets | 311845353238 | 25807G / 09/2024 |
People's Choice Women's Daily | 30 tablets | 311845486882 | 25807G / 09/2024 A25807G / 09/2024 B25807G / 09/2024 C25807G / 09/2024 D25807G / 09/2024 |
To date, no illnesses related to these products have been reported. No other People's Choice and Healthy Sense products are affected by this recall. If customers have product affected by this voluntary recall, they should discard it immediately.
For any additional information, please call Customer Care 1-888-860-5376, Monday through Friday 9:00 AM to 5:00 PM EST. We regret any inconvenience this may cause.
Contact: | [email protected] |
SOURCE Mason Vitamins
These press releases may also interest you
|
News published on and distributed by: