GSK ships 2018-19 seasonal influenza vaccines for US market
PHILADELPHIA, Aug. 8, 2018 /PRNewswire/ -- GSK today announced it will begin shipping its quadrivalent influenza vaccines to US healthcare providers and pharmacies for the 2018-19 flu season, immediately following licensing and lot-release approval from the US Food and Drug Administration's (FDA) Center for Biologics Evaluation and Research.
"The 2017-18 flu season was an important reminder that the flu is a serious and unpredictable disease," said Dr. Leonard Friedland, VP, director of Scientific Affairs and Public Health, Vaccines, North America. "Annual vaccination can help prevent flu illnesses and flu-related hospitalizations and reduce the severity of the disease. The more people of all ages who are immunized, the less chance the virus has to spread and the better we can help protect against the flu this season."
The US Centers for Disease Control and Prevention (CDC) estimates that flu vaccination during the 2016-17 flu season prevented an estimated 5.29 million illnesses, 2.64 million medical visits and 84,700 hospitalizations associated with flu, underscoring the benefits of current vaccines.[i] Yet, according to the CDC, approximately 80 percent of flu-related deaths in children during the 2017-18 season occurred in children who were not vaccinated against the flu that season.[ii]
"The flu vaccine is the one vaccine that people of almost all ages should receive annually," said Patrick Desbiens, Senior Vice President, US Vaccines. "GSK is committed to helping healthcare professionals provide flu vaccinations across the lifespan. Beginning with the 2018-19 flu season, we can now offer two vaccines that enable providers to vaccinate all of their recommended patients aged 6 months and older with the same vaccine dose."
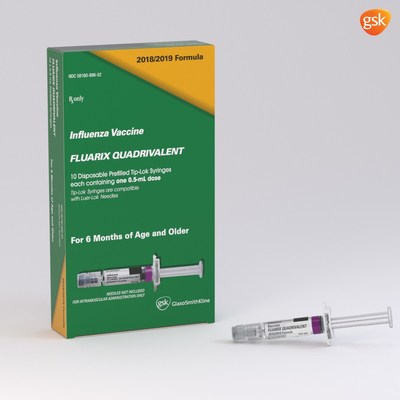
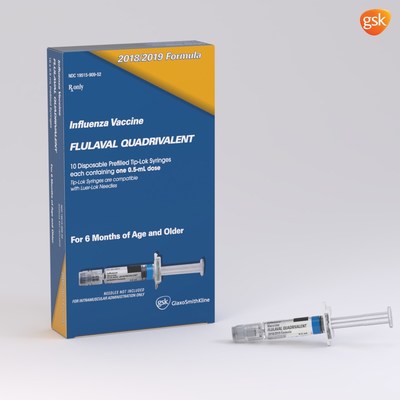
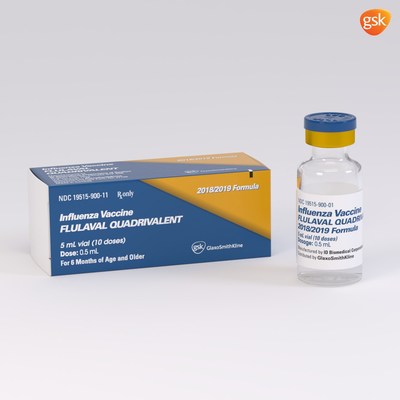
GSK expects to supply up to 40 to 45 million total doses across both of its vaccines for the US market in the 2018-19 season, the highest volume of doses planned for distribution by GSK to date. FLULAVAL QUADRIVALENT will be available in a 5mL, multidose vial containing 10 doses (0.5mL each) and a 0.5mL, single-dose, prefilled syringe. FLUARIX QUADRIVALENT will be available in a 0.5mL, single-dose, prefilled syringe.
For the 2018-19 flu season, the World Health Organization (WHO) and FDA's Vaccines and Related Biological Products Advisory Committee recommended including the A/Michigan/45/2015 (H1N1) pdm09-like virus, A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus and B/Colorado/06/2017-like virus, with the addition of B/Phuket/3073/2013-like virus for the quadrivalent vaccine.[iii],[iv]
About seasonal influenza
Seasonal influenza (the "flu") is a contagious respiratory illness, caused by flu viruses.[v] There are two main types of flu viruses, A and B, that spread between people and can cause mild to severe illness.[vi] Most flu activity in the US occurs from October through May, and it usually peaks between December and February.[vii]
While anyone can get the flu, it can be particularly serious for young children, older people, pregnant women and people with certain health conditions, such as asthma.[viii] According to the CDC, the best tool available to help protect yourself and those around you against the flu is to get vaccinated. The more people who are vaccinated, the less chance the virus has to spread.[ix] The CDC recommends that all people over the age of 6 months get vaccinated against the flu annually.[x]
For more information about the flu, visit flu.gsk.com.
Indication for FLUARIX QUADRIVALENT and FLULAVAL QUADRIVALENT
FLUARIX QUADRIVALENT and FLULAVAL QUADRIVALENT are vaccines indicated for active immunization for the prevention of disease caused by influenza A subtype viruses and type B viruses contained in the vaccines. FLUARIX QUADRIVALENT and FLULAVAL QUADRIVALENT are approved for use in persons aged 6 months and older.
Important Safety Information for FLUARIX QUADRIVALENT and FLULAVAL QUADRIVALENT
- Do not administer FLUARIX QUADRIVALENT or FLULAVAL QUADRIVALENT to anyone with a history of severe allergic reactions (eg, anaphylaxis) to any component of the vaccine, including egg protein, or following a previous dose of any influenza vaccine
- If Guillain-Barré syndrome has occurred within 6 weeks of receipt of a prior influenza vaccine, the decision to give FLUARIX QUADRIVALENT or FLULAVAL QUADRIVALENT should be based on careful consideration of the potential benefits and risks
- Syncope (fainting) can occur in association with administration of injectable vaccines, including FLUARIX QUADRIVALENT or FLULAVAL QUADRIVALENT. Procedures should be in place to avoid falling injury and to restore cerebral perfusion following syncope
- If FLUARIX QUADRIVALENT or FLULAVAL QUADRIVALENT is administered to immunosuppressed persons, including individuals receiving immunosuppressive therapy, the immune response may be lower than in immunocompetent persons
- In clinical trials with FLUARIX QUADRIVALENT in adults, the most common solicited local adverse reaction was pain and the most common systemic adverse reactions were muscle aches, headache, and fatigue. In children 6 through 35 months of age, the most common solicited local adverse reactions were pain and redness and the most common systemic adverse reactions were irritability, loss of appetite, and drowsiness. In children 3 through 17 years of age, the solicited local adverse reactions were pain, redness, and swelling. In children 3 through 5 years of age, the most common systemic adverse reactions were drowsiness, irritability, and loss of appetite. In children 6 through 17 years of age, the most common systemic adverse reactions were fatigue, muscle aches, headache, arthralgia, and gastrointestinal symptoms. (See Adverse Reactions section of the Prescribing Information for FLUARIX QUADRIVALENT for other potential adverse reactions and events)
- In clinical trials with FLULAVAL QUADRIVALENT in adults, the most common solicited local adverse reaction was pain and the most common solicited systemic adverse reactions were muscle aches, headache, fatigue, and arthralgia. In children 6 through 35 months of age, the most common solicited local adverse reaction was pain and the most common solicited systemic adverse reactions were irritability, drowsiness, and loss of appetite. In children 3 through 17 years of age, the most common solicited local adverse reaction was pain. In children 3 through 4 years of age, the most common solicited systemic adverse reactions were irritability, drowsiness, and loss of appetite. In children 5 through 17 years of age, the most common solicited systemic adverse reactions were muscle aches, fatigue, headache, arthralgia, and gastrointestinal symptoms. (See Adverse Reactions section of the Prescribing Information for FLULAVAL QUADRIVALENT for other potential adverse reactions and events)
- Vaccination with FLUARIX QUADRIVALENT or FLULAVAL QUADRIVALENT may not result in protection in all vaccine recipients
Please see full Prescribing Information for FLUARIX QUADRIVALENT and for FLULAVAL QUADRIVALENT.
GSK - a science-led global healthcare company with a special purpose: to help people do more, feel better, live longer. For further information please visit www.gsk.com
|
GSK enquiries: |
|||
|
US Media enquiries: |
Sean Clements |
+1 215 347 9274 |
(Philadelphia) |
|
Gwynne Oosterbaan |
+1 215 751 7468 |
(Philadelphia) | |
|
Analyst/Investor enquiries: |
Sarah Elton-Farr |
+44 (0) 20 8047 5194 |
(London) |
|
Gary Davies |
+44 (0) 20 8047 5503 |
(London) | |
|
James Dodwell |
+44 (0) 20 8047 2406 |
(London) | |
|
Jeff McLaughlin |
+1 215 751 7002 |
(Philadelphia) | |
Cautionary statement regarding forward-looking statements
GSK cautions investors that any forward-looking statements or projections made by GSK, including those made in this announcement, are subject to risks and uncertainties that may cause actual results to differ materially from those projected. Such factors include, but are not limited to, those described under Item 3.D 'Principal risks and uncertainties' in the company's Annual Report on Form 20-F for 2017.
Registered in England & Wales:
No. 3888792
Registered Office:
980 Great West Road
Brentford, Middlesex
TW8 9GS
[i] Centers for Disease Control and Prevention. Estimated Influenza Illnesses, Medical visits, and Hospitalizations Averted by Vaccination in the United States. Available at: https://www.cdc.gov/flu/about/disease/2016-17.htm. Accessed July 2018.
[ii] Centers for Disease Control and Prevention. CDC Reported Flu Deaths in Children Exceeds Seasonal High. Available at: https://www.cdc.gov/flu/spotlights/reported-flu-children-deaths.htm. Accessed July 2018.
[iii] World Health Organization. Recommended composition of influenza virus vaccines for use in the 2018-2019 northern hemisphere influenza season. Available at: http://www.who.int/influenza/vaccines/virus/recommendations/2018_19_north/en/. Accessed July 2018.
[iv] US Food and Drug Administration. Global Surveillance and Virus Characterization. Available at: https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/UCM601883.pdf. Accessed July 2018.
[v] Centers for Disease Control and Prevention. Key Facts about Influenza (Flu). Available at: http://www.cdc.gov/flu/keyfacts.htm. Accessed July 2018.
[vi] Centers for Disease Control and Prevention. Types of Influenza Viruses. Available at: https://www.cdc.gov/flu/about/viruses/types.htm. Accessed July 2018.
[vii] Centers for Disease Control and Prevention. The Flu Season. Available at: https://www.cdc.gov/flu/about/season/flu-season.htm. Accessed July 2018.
[viii] Centers for Disease Control and Prevention. People at High Risk of Developing Flu?Related Complications. Available at: https://www.cdc.gov/flu/about/disease/high_risk.htm. Accessed July 2018.
[ix] Centers for Disease Control and Prevention. What Would Happen If We Stopped Vaccinations? https://www.cdc.gov/vaccines/vac-gen/whatifstop.htm. Accessed July 2018.
[x] Centers for Disease Control and Prevention. Vaccination: Who Should Do It, Who Should Not and Who Should Take Precautions. Available at: https://www.cdc.gov/flu/protect/whoshouldvax.htm. Accessed July 2018.
SOURCE GSK
These press releases may also interest you
|
News published on and distributed by:



