Subjects: PDT, TDS, FVT
Tyber Medical Announces the Limited Release of the Sterile Comprehensive Forefoot Procedure Kit
BETHLEHEM, Pa., March 21, 2018 /PRNewswire/ -- Tyber Medical, LLC, a privately held company focusing on developing innovative medical devices for private label opportunities and advancing the science of bioengineered surfaces, announces the limited release of the Forefoot Procedure Kit.
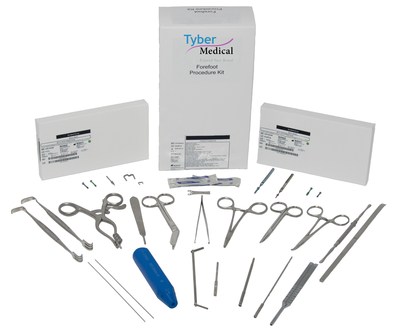
Tyber Medical is excited to announce the limited release of the Forefoot Procedure Kit. This innovative system is the first efficiency kit on the market to incorporate room setup disposables, instruments for soft tissue, bone and implant preparation, and the corresponding implants. The system configuration allows for flexibility in the treatment of a wide range of fixation procedures including bunion corrections, hammertoes, Aikens as well as Weil's osteotomies. This unique one of kind system is provided sterile to increase efficiency in the Operating Room.
The complete kit eliminates the necessity for cleaning, decontamination, sterilization, and reprocessing thereby reducing direct and indirect costs while accelerating room turnover to create a more efficient center. All implants are sterile, ready-to-use, and include headed or headless screws, TyFixtm hammertoe screws, snap-off screws, as well as NiTiNOL staples in various sizes to suit any type of Forefoot procedure.
Catye Mullens, a Certified Surgical Technician in Materials Management at Wilshire Surgery Center and a member of the design team stated, "The Forefoot Procedure Kit is a time saver for OR setups and comes with all the fundamental items we need and nothing we don't. We also appreciate the efficient space management design which minimizes shelf requirements for our surgery center." Donna Faillace, a Certified Surgical Technician at Providence Health Center commented "The Forefoot Procedure Kit makes it so easy to set up for surgeries knowing everything our surgical team and center needs is included. It decreases turnover time because you're not waiting for instruments to process and the surgeon is pleased because he has consistent equipment available for every case."
"The Forefoot Procedure Kit was designed to be an inclusive system delivering a procedure in a box while allowing the surgeon flexibility to create any type of Forefoot reconstructive procedure. The kit aligns efficiency and effectiveness together to offer a solution for cost reduction, time efficiencies, and better organization of pre-operative planning," commented Jeff Tyber, CEO of Tyber Medical.
Tyber Medical continues to focus on systems designed to drive pre-operative, intra-operative, and post-operative efficiency, while embracing system improvements that could lead to a lower rate of surgical field contamination and subsequent patient infections. Please visit Tyber Medical's Booth #333, at ACFAS in Nashville, TN where the company will showcase the Forefoot Procedure Kit and recently released TyFixtm System as well as other innovative products available for private labeling.
About Tyber Medical, LLC.
Tyber Medical, LLC is an orthopedic device manufacturer providing rapid access to portfolio enhancing, regulatory approved, orthopedic implants within the spine and extremity/trauma markets. While focusing on rapid product commercialization, the company distributes products via private labeling; releasing 14 spine and 25 extremity/trauma systems since its founding in 2012. Tyber Medical utilizes the differentiated, bioengineered technology such as TyPEEK® osteoconductive coatings and BioTytm, a new antimicrobial nano-textured surface modification to enhance the company's growing portfolio.
Contact:
Eric Dickson
83 South Commerce Way, Suite 310
Bethlehem PA 18017
(610) 849-1710
[email protected]

SOURCE Tyber Medical, LLC
These press releases may also interest you
|
News published on and distributed by:



