Subject: PDT
VivaLNK Announces Developers Program for New eSkin Technology
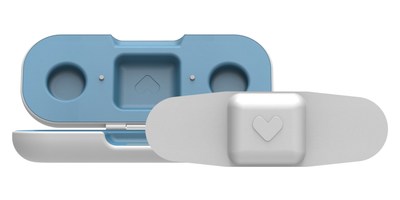
SANTA CLARA, Calif., Dec. 17, 2017 /PRNewswire/ -- VivaLNK announces Vital Scout, a wellness assessment patch for medical grade accuracy not seen before with wrist worn devices. The Vital Scout uses electrocardiography (ECG) and 3D accelerometer sensors to monitor Heart Rate (HR), Respiration Rate (RR), and Heart Rate Variability (HRV) with specialized algorithms to determine your level of stress, recovery, duration and quality of sleep as well as activity/exercise intensity.
Vital Scout uses ECG sensors to provide more accurate readings of HR & RR in comparison to wrist-worn devices, which commonly use optical photoplethysmography (PPG) sensors. What's really unique about the Vital Scout patch is the lifestyle management application, which provides actionable insights. The company is targeting this device to be sold to wellness professionals with the goal of making changes to improve health of their clients. Unlike wrist worn devices, the patch could be worn for shorter periods of time (24-72 hours) yet provide more accurate meaningful data to review and take action.
No need to be hooked up to wires or wear tight straps across the body, about the size of a band-aid the Vital Scout patch is the smallest wearable patch currently out on the market. Worn over the chest with adhesives, users can easily go about their daily activities with comfort. With up to 72 hr of continuous monitoring wellness professionals can work with their clients to (1) pinpoint areas of improvement, (2) set wellness goals, (3) follow up to see if the change in habits are effective and (4) encourage behavioral changes.
VivaLNK has been working closely with health analytics companies, and cardiac specialists over the years to develop Vital Scout, which would be available for wellness companies and professionals by the end of Q1 2018.
Due to great interest for reliable biometric data VivaLNK is open to connecting with businesses in the Health and Wellness space by creating a developers program so that developers can interact with Vital Scout data in their own application, products and services. To learn more about their API and enroll into VivaLNK's Developers Program you can visit the following link Link To Enroll into the Vital Scout's Developers Program.
Also for those attending CES in Las Vegas, VivaLNK will be revealing Vital Scout's new design and features. VivaLNK invites visitors to see the live demo of Vital Scout taking place in Sands/Venetian Expo Hall A-D Booth #43740. Link to Schedule Live Demo of Vital Scout at CES
About VivaLNK
VivaLNK, Inc. utilizes their patented eSkinTM technology, which is a circuit board that is as thin as your skin. It's a thin electronic substrate comfortable to wear, breathable, non-invasive and used as a platform for sensor integration and data services. VivaLNK is also the company behind Fever Scout the wearable smart thermometer patch that continuously sends data to your smartphone and alerts you of fever spikes. For more information, visit www.vivalnk.com and follow us on Twitter at @Viva_Lnk.
Contact:
Lucia Nguyen
1-408-829-8561
[email protected]
SOURCE VivaLNK
These press releases may also interest you
|
News published on and distributed by:



