Subject: CXP
HeartStitch® Leading the growth of PFO Closure in Italy
MILAN, Dec. 11, 2017 /PRNewswire/ -- Kardia announced expansion plan of the NobleStitchtm EL at a recent meeting organized by Professor Francesco Romeo at Policlinico Tor Vergata in Rome where Prof. Achille Gaspardone performed a successful NobleStitchtm EL live case that was transmitted to the attendees in Ayla Magna, Rome, Italy.
Prof. Gaspardone, who recently presented 2 studies (The Italian/ Swedish Registry of NobleStitchtm EL and his Single Center Study of PFO Closure with NobleStitchtm EL) and has the largest experience in Italy, more than 80 cases, performed the live case on a 37 year old female patient who suffered from migraines with aura and had a previous cerebral infarct due to PFO. The attendees, many who had not seen a NobleStitchtm EL procedure, provided very positive feedback and significant interest in using the NobleStitchtm EL.
Roberto Riva, manager of Kardia, stated, "over the last 18 months we have seen strong growth in the market for PFO closure. Initially we rolled out the product to a few of our key centers to develop the market for the NobleStitchtm and to accumulate Italian data. We have been astonished at the willingness by physicians to start with the product and even more their desire to adopt the NobleStitchtm as their first choice for PFO closure. The product is so intuitive to both physicians and patients that we see the patients specifically asking for the product over the traditional umbrella devices. In my 20 years in the medical instrument sales I have not had a product like this. Our initial forecasts were conservative and we quickly requested more product from HeartStitch®. We are already in 17 centers in Italy, trained more than 24 cardiologists and performed more than 220 procedures. Our new market plan for 2018 with HeartStitch® will drive the "Sutures the Future" as we look to cover the remaining PFO centers throughout Italy with the NobleStitchtm EL and look forward to the release of the HeartStitch® Transapical and Mitral devices that our customers are awaiting."
Prof. Gaspardone also commented, "We have worked with the device for 18 months and developed a very simple technique that can be trained and adopted quickly. Personally I have really been impressed by the simplicity of the system and the clinical data, which has demonstrated to me that it is the first choice and the future of PFO closure. This live case demonstrates how simple the procedure has become and the efficacy of the technique. It has and continues to be my first choice and my patients also ask for it. I have an ever-growing waiting list of patients that we will continue to treat with this revolutionary technology."
Dirk Segers, VP of European Sales and Marketing noted, "Italy was our first nationwide expansion in the European Union and is a critical part of our business model. Physicians and patients in Italy have been very responsive to the NobleStitchtm EL as their first choice for PFO closure. It's intuitive to both physicians and patients who suffer from issues associated with PFO. We did not expect the rapid uptake and adoption of the NobleStitchtm EL to be so quick. In my career I have not seen such a response to a medical device."
Professor Anthony Nobles, Inventor of the NobleStitchtm EL and Chairman, CEO and Chief Clinical Officer of HeartStitch® which manufactures and distributes the devices, commented, "We are very excited about the performance of Kardia and their sub-distributors throughout Italy as well as the physicians and patients that request our NobleStitchtm device for PFO closure. We are pleased with the increase demand worldwide since the publication of recent positive trials including the presentation of the NobleStitchtm data from Italy and Sweden. We look to replicate the success of our Italian experience throughout Europe and then the US."
About PFO Closure
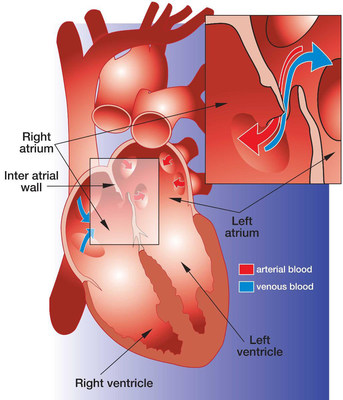
A PFO is a relatively common heart defect characterized by an unsealed tunnel between the right and left atria of the heart. This defect has been known to be present in anywhere between 27%-38% of people. However, in a number of cases, it is benign.
The PFO is formed as a trace of the fetal circulation. When the chambers of a human heart begin to develop, a communication is made between the right and left atria, allowing blood to flow directly from the venous circulation to the arterial circulation, circumventing the non-functioning fetal lungs. Following birth, the pressure differential between the right and left atria changes with newly operational blood flow to the fully functioning lungs. Because of this, the communication eventually closes completely within the first few months.
However, in some patients, the foramen ovale fails to seal and remains "patent". In patients with a Patent Foramen Ovale (PFO), the communication can reopen under elevated atrial pressure, such as coughing, or straining.
A key issue with PFO is that it gives a pathway for blood clots to pass directly to the arterial circulation without being filtered out by the capillary bed of the lungs. A PFO can also let deoxygenated blood and certain chemicals cross over to the arterial side. The presence of a PFO has been linked to a number of clinical issues, mainly strokes, migraines and chronic fatigue. Developments are being made to solidify the link between PFO and strokes or migraines, and to identify patients that would benefit from PFO closure.
About HeartStitch®
HeartStitch® Inc. was founded by Prof. Anthony Nobles with the intent of leveraging its technologies in the structural heart marketplace. HeartStitch® is focused on innovative suture-based systems for remotely providing suture repair of structural heart defects and other vascular structures.
The HeartStitch® TA and HeartStitch® MR are FDA cleared for vascular suturing in the United States. HeartStitch® manufactures and markets the NobleStitchtm EL under exclusive license from Nobles Medical technologies II, Inc. NobleStitchtm EL is FDA cleared for vascular suturing in the United States and CE Marked for cardio-vascular suturing and PFO closure in the European Union and the Republic of Kazakhstan, respectively.
HeartStitch® is a registered trademark of HeartStitch® Inc.
HeartStitch® TA
Covered by or for use under U.S. and international patents including one or more of U.S. Patent Nos. 5860990, 6117144, 6245079, 6551331, 6562052, 6733509, 7004952, 7090686, 7803167, 8197497, 8348962, 8469975, 8496676, and 8709020.
HeartStitch® MR
Covered by or for use under U.S. and international patents including one or more of U.S. Patent Nos. 5860990, 6117144, 6245079, 6551331, 6562052, 6733509, 7004952, 7090686, 7803167, 8197497, 8348962, 8469975, 8496676, 8709020, and 8771296.
For more on HeartStitch® visit http://www.heartstitch.com
For more information, please contact shareholder representatives:
USA
Dru Dobbs
P. +1 714 427 6348
F. +1 714 427 6343
[email protected]
In Kazakhstan
Kazbek Aubakirov
P. +7 777 5009005
[email protected]
About Nobles Medical Technology II
Nobles Medical Technology II, Inc. was founded by Prof. Anthony Nobles with the intent of leveraging its technologies in the PFO, ASD-closure, and cardiovascular-suturing marketplace. The company does business under the name of Nobles Medical II (NMT II). Initial efforts of the company have been focused in Europe on the innovative suture-based PFO closure system for closing the Patent Foramen Ovale (PFO), a tunnel between the right and left atria of the heart.
The NobleStitchtm is approved for PFO Closure and Cardiovascular suturing in the European Union.
The NobleStitchtm EL is FDA cleared for Vascular and Cardiovascular suturing in the United States. Further information including warnings and precautions can be found in the instructions for use.
NobleStitchtm EL is distributed worldwide by HeartStitch®, Inc. (HeartStitch® is a registered trademark of HeartStitch, Inc.).
NobleStitchtm EL for PFO closure
Covered by or for use under U.S. and international patents including one or more of U.S. Patent Nos. 5860990, 6117144, 6245079, 6551331, 6562052, 6733509, 7004952, 7090686, 7803167, 8197497, 8197510, 8246636, 8348962, 8372089, 8469975, 8496676, 8709020, and 9131938.
For more on Nobles Medical Technologies II visit
http://www.noblesmed2.com.
Dru Dobbs
P. +1 714 427 6348
F. +1 714 427 6343
[email protected]

SOURCE HeartStitch® Inc.
These press releases may also interest you
|
News published on and distributed by:



