Subjects: NPT, SVY, BFA, TRI
FH Foundation Announces Consensus Statement On Genetic Testing For Familial Hypercholesterolemia (FH)
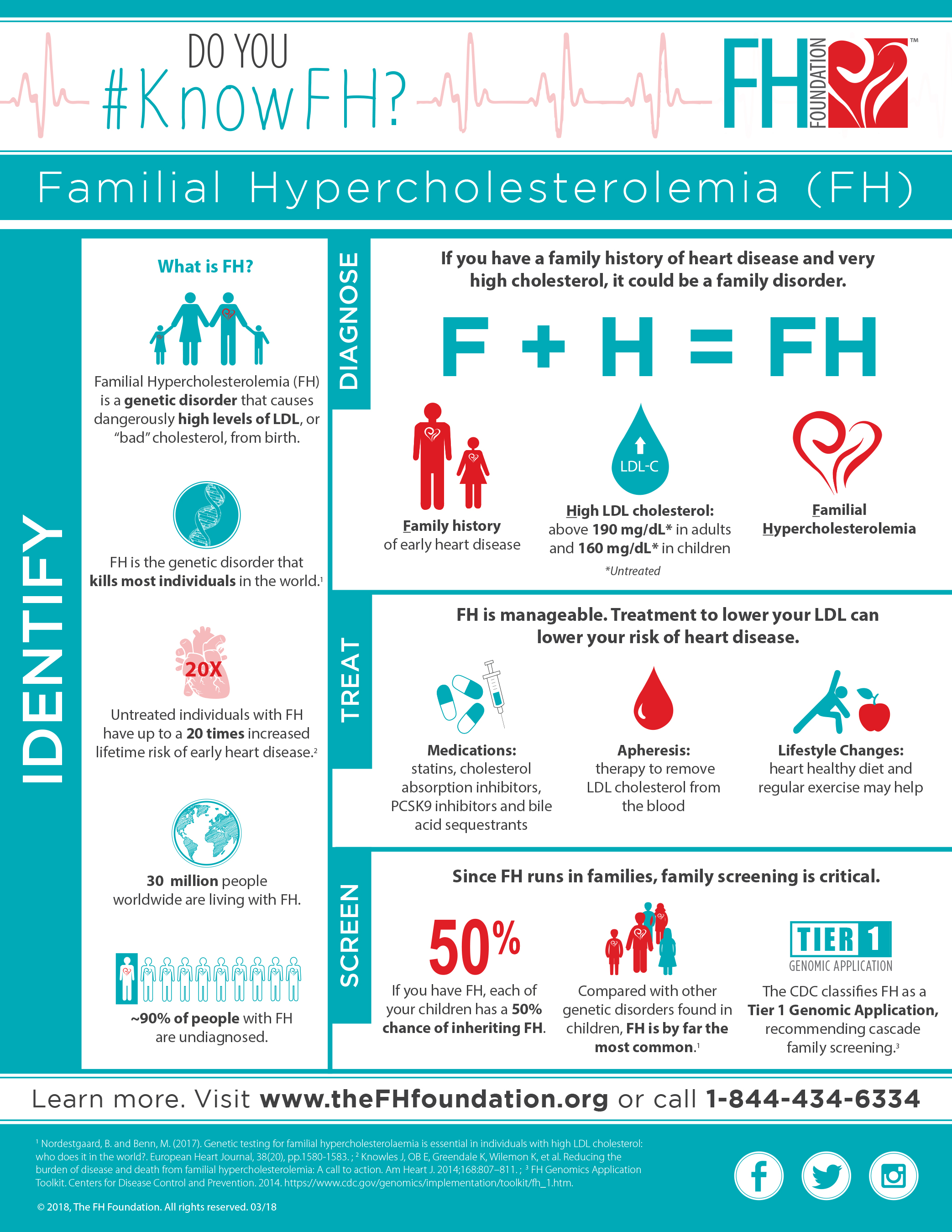
PASADENA, Calif., July 30, 2018 /PRNewswire/ -- The FH Foundation announced that an important consensus statement on diagnosing familial hypercholesterolemia (FH), a common life-threatening genetic condition, was published online today in the Journal of the American College of Cardiology. Authored by an international panel of 30 cardiovascular, lipid, genetics and advocacy experts and led by the FH Foundation, the statement recommends that genetic testing be offered to diagnose both individuals with FH and their relatives. FH genetic mutations lead to heart disease and stroke risk during the prime of life. Genetic testing of at-risk individuals holds the promise of accelerating the identification of over 30 million people worldwide born with FH, 90 percent of whom are currently undiagnosed, to ensure early treatment.i,ii
Experience the interactive Multichannel News Release here: https://www.multivu.com/players/English/8365851-fh-foundation-familial-hypercholesterolemia-know-fh/
"Diagnosing and treating FH in childhood reduces the risk of early heart disease by about 80 percent, which is why it's so important to find families with FH and especially children who have this invisible, life-threatening genetic disorder," said Amy C. Sturm, M.S., C.G.C., Genomic Medicine Institute, Geisinger, and co-author of the paper. "In addition, understanding the exact genetic mutation can better inform the initiation and treatment of FH with more intense lipid-lowering therapies and ultimately improve outcomes."
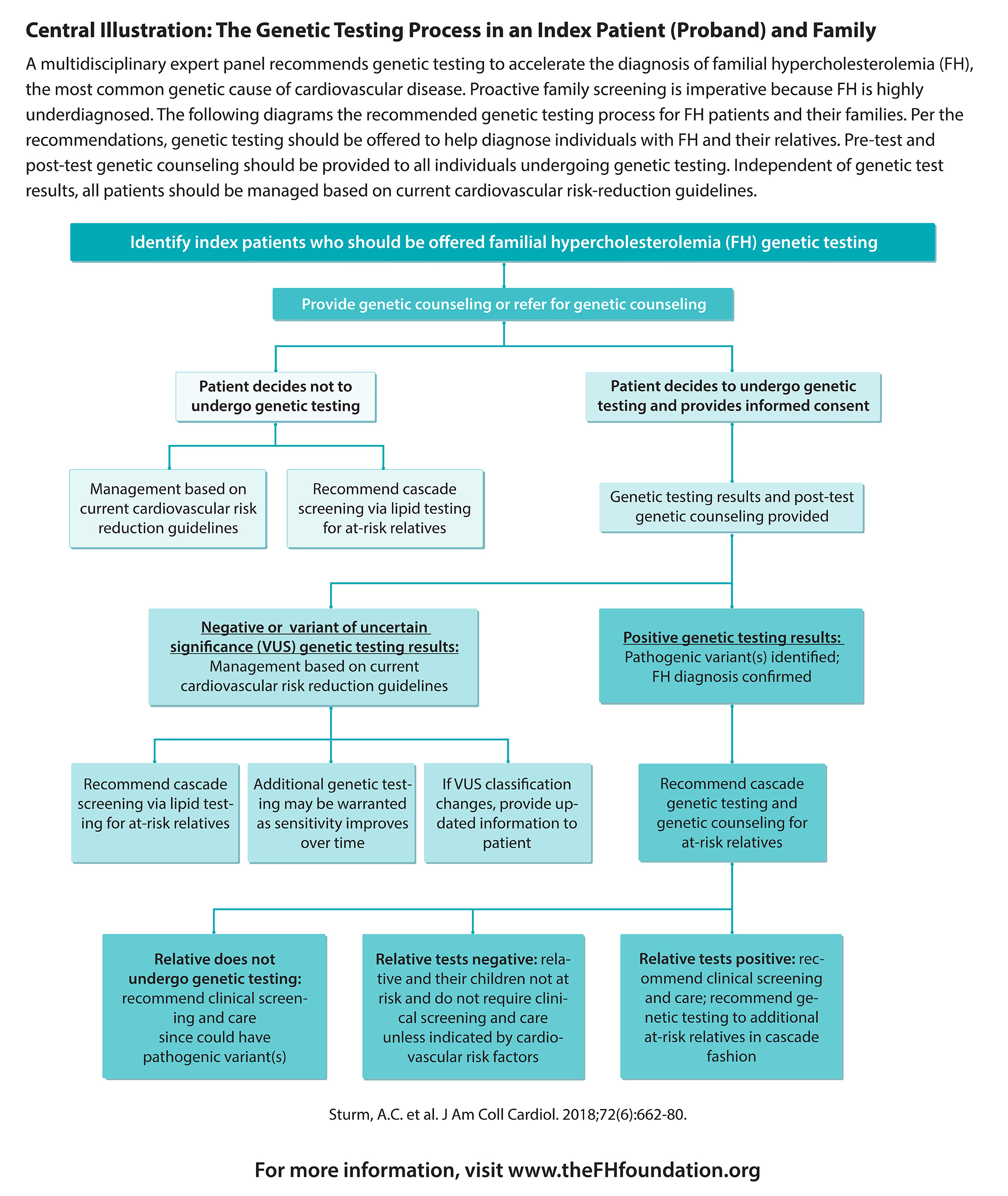
The consensus statement recommends that FH genetic testing be offered for patients with very high LDL cholesterol and a positive family history of high cholesterol or early heart attack. Such individuals have definite or probable FH and should be tested to detect variants in three genes that account for the majority of FH cases and can increase a person's risk for coronary artery disease (CAD): Low Density Lipoprotein Receptor (LDLR), Apolipoprotein B (APOB) or Proprotein Convertase Subtilisin/Kexin type 9 (PCSK9). At-risk family members of those who test positive should also be tested. Although FH can be diagnosed using clinical criteria, the statement recommends that genetic testing be used to provide:
- A definitive diagnosis
- Proactive family screening
- Timely initiation and intensification of therapies
- Improved risk stratification
FH is an autosomal dominant inherited disorder resulting in significantly elevated low-density lipoprotein (LDL) cholesterol. Individuals with FH are at a 6-20 times increased lifetime risk of heart disease, and each first-degree relative has a 50 percent chance of inheriting this life-threatening genetic disorder.
"In this era of precision medicine, genetic testing is an important tool to identify people at high risk for FH and guide the management of their LDL cholesterol to reduce long-term morbidity and mortality from early and aggressive CAD," said Daniel J. Rader, M.D., chair of the department of Genetics in the Perelman School of Medicine at the University of Pennsylvania, Chief Scientific Advisor of The FH Foundation and co-author of the paper. "Physicians should entertain the diagnosis of FH in their patients who have a family history of early heart disease and/or high LDL cholesterol, and consider offering a genetic test in those who may have FH."
The Centers for Disease Control Office of Public Health Genomics includes FH testing as a Tier 1 genomic application, recognizing the positive public health impact of FH family screening.iii
"Familial hypercholesterolemia is perhaps the biggest untapped opportunity to prevent premature cardiovascular disease. It is vital that healthcare providers and individuals understand the importance of differentiating this genetic condition from life-style induced high cholesterol," said Katherine Wilemon, founder and CEO of the FH Foundation and co-author of the paper. "The FH Foundation convened experts from multiple fields and around the world to review the benefits and limitations of using genetic testing as one means of improving the diagnosis and care of FH."
Genetic testing can be beneficial in confirming a diagnosis of FH. However, FH can be diagnosed without a genetic test. Furthermore, a negative genetic test result does not eliminate the clinical diagnosis of FH. In summary, the panel concluded that patients with a family history of early heart disease and substantially elevated LDL cholesterol should be screened for FH and then offered a genetic test to confirm the diagnosis.
To learn more:
- Join the Tweet Chat on Thurs., August 16 at 3:00 pm EDT. Visit www.twitter.com and type the hashtag #FHCantWait. Follow the FH Foundation on Twitter @TheFHFoundation.
- Visit TheFHFoundation.org for more information on FH, including resources for physicians and patients, and information on genetic testing.
About Familial Hypercholesterolemia (FH)
FH is the most common genetic cause of early, life-threatening cardiovascular disease. FH is a global public health concern that affects over 30 million people worldwide and more than 1.3 million people in the U.S., but less than 10 percent are diagnosed.iv FH causes high LDL cholesterol from birth and is the cause for 20 percent of early heart attacksv (heart attacks that occur in the 20's, 30's and 40's). Early and significant reduction of LDL cholesterol is key to successful management of FH, which requires lifelong treatment.
About the FH Foundation
The FH Foundation is a leading research and advocacy non-profit organization focused on reducing heart disease by driving scientific understanding and evidence-based care of FH. The mission of The FH Foundation is to save lives by contributing to scientific research that leads to greater understanding and improved diagnosis and treatment of familial hypercholesterolemia worldwide. Please visit www.TheFHFoundation.org for more information.
i Nordestgaard BG, Benn M. Genetic testing for familial hypercholesterolaemia is essential in individuals with high LDL cholesterol: who does it in the world? Eur Heart J 2017;38:1580?3.
ii Nordestgaard BG, Chapman MJ, Humphries SE, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J 2013;34: 3478?90a.
iii FH Genomics Application Toolkit. Centers for Disease Control and Prevention. 2014. https://www.cdc.gov/genomics/implementation/toolkit/fh_1.htm.
iv Knowles J, et al. Reducing the burden of disease and death from familial hypercholesterolemia: A call to action. Am Heart J.2014;168:807-811.
v Hopkins P, Toth P. Familial hypercholesterolemia: prevalence, genetics, diagnosis and screening recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011 June;5(3 Suppl):S9?17.
SOURCE FH Foundation
These press releases may also interest you
|
News published on and distributed by:



