Subject: TDS
NobleStitchtm EL PFO Closure Highlighted at CSI Congress with Prof. Horst Sievert Performing his 100th NobleStitch Case on Opening Day
FRANKFURT, Germany, July 10, 2018 /PRNewswire/ -- NMT2 was again a featured participant at the 2018 CSI Congress in Frankfurt, Germany. Two live case procedures of PFO closure using the NobleStitchtm EL were broadcast to congress attendees. The cases were presented by Prof Dr. Horst Sievert, (Director and Founder CVC Frankfurt, Germany; Board of Directors CSI), and Prof Anthony Nobles, CEO and Inventor of the NobleStitchtm EL. The both cases highlighted the percutaneous suture-based closure system on patients suffering from cryptogenic stroke.
The result of both procedures was successful closure using the suture-based system whose less invasive percutaneous treatment is rapidly becoming the first choice for physicians worldwide. Expanding use of the NobleStitchtm EL is garnering more data on the safety and efficacy of the device. A recent publication of Italian clinical study data of 200 patients proved the successful closure results, along with data showing no atrial fibrillation, and no need for long term therapies post procedure and a greater safety profile than traditional metal umbrella devices.
Prof Sievert has been working with the NobleStitchtm EL in his clinic since 2016 and has successfully treated more than 100 patients. This is the third consecutive year that he has chosen to present the device in the Live Case format at the CSI Frankfurt congress owing to his belief in the device as highly successful in PFO closure for his patients. During the live case Prof. Sievert commented, "The recent publications showed that the NobleStitchtm closure rates are equivalent to the standard devices and have had no recorded atrial fibrillations in over 1,100 patients. Also, the device is very simple to use and leaves nothing on the left side of the heart, which is a big advantage over traditional devices."
Professor Anthony Nobles, Inventor of the NobleStitchtm EL and Chairman, CEO and Chief Clinical Proctor of NMT2 who few into Frankfurt for the Live Cases has been traveling throughout Europe proctoring physicians in the use of the NobleStitchtm.
Prof Nobles noted, "We have been working with Prof. Sievert and CSI for several years and his enthusiasm for this procedure has grown to become a regular monthly event at CVC with many patients (sometimes as many as 12 in a single day), several requesting the NobleStitchtm specifically and traveling from across Germany and abroad to be treated with the NobleStitchtm. For NMT2 Prof. Sievert and CVC has become a key training center for other proctors to learn the NobleStitch more rapidly because of the high volume of cases performed in a single day each month."
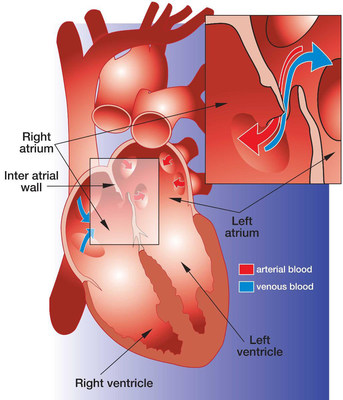
About PFO Closure
A PFO is a relatively common heart defect characterized by an unsealed tunnel between the right and left atria of the heart. This defect has been known to be present in anywhere between 27%-38% of people. However, in a number of cases, it is benign.
The PFO is formed as a trace of the fetal circulation. When the chambers of a human heart begin to develop, a communication is made between the right and left atria, allowing blood to flow directly from the venous circulation to the arterial circulation, circumventing the non-functioning fetal lungs. Following birth, the pressure differential between the right and left atria changes with newly operational blood flow to the fully functioning lungs. Because of this, the communication eventually closes completely within the first few months.
However, in some patients, the foramen ovale fails to seal and remains "patent." In patients with a Patent Foramen Ovale (PFO), the communication can reopen under elevated atrial pressure, such as coughing, or straining.
A key issue with PFO is that it gives a pathway for blood clots to pass directly to the arterial circulation without being filtered out by the capillary bed of the lungs. A PFO can also let deoxygenated blood and certain chemicals cross over to the arterial side. The presence of a PFO has been linked to a number of clinical issues, mainly strokes, migraines and chronic fatigue. Developments are being made to solidify the link between PFO and strokes or migraines, and to identify patients who would benefit from PFO closure.
About Nobles Medical Technology II
Nobles Medical Technology II, Inc. was founded by Prof. Anthony Nobles with the intent of leveraging its technologies in the PFO, ASD-closure, and cardiovascular-suturing marketplace. The company does business under the name of Nobles Medical II (NMT II). Initial efforts of the company have been focused in Europe on the innovative suture-based PFO closure system for closing the Patent Foramen Ovale (PFO), a tunnel between the right and left atria of the heart.
The NobleStitchtm is approved for PFO Closure and Cardiovascular suturing in the European Union.
The NobleStitchtm EL is FDA cleared for Vascular and Cardiovascular suturing in the United States. Further information including warnings and precautions can be found in the instructions for use.
NobleStitchtm EL is distributed worldwide by HeartStitch®, Inc. (HeartStitch® is a registered trademark of HeartStitch, Inc.).
NobleStitchtm EL for PFO closure
Covered by or for use under U.S. and international patents including one or more of U.S. Patent Nos. 5860990, 6117144, 6245079, 6551331, 6562052, 6733509, 7004952, 7090686, 7803167, 8197497, 8197510, 8246636, 8348962, 8372089, 8469975, 8496676, 8709020, and 9131938.
HeartStitch® manufactures and markets the NobleStitchtm EL under exclusive license from Nobles Medical Technologies II, Inc. NobleStitchtm EL is FDA cleared for vascular suturing in the United States and CE Marked for cardio-vascular suturing and PFO closure in the European Union and the Republic of Kazakhstan, respectively.
For more on Nobles Medical Technologies II visit

SOURCE Nobles Medical Technology II, Inc.
These press releases may also interest you
|
News published on and distributed by:



