Subjects: SVY, TDS, CXP, FVT
CleanCisiontm System Earns CE Mark Approval
MINNEAPOLIS, June 12, 2018 /PRNewswire/ -- APIC 2018 -- Prescient Surgical today announced it received CE Mark approval for its first-in-class, advanced technology CleanCisionTM which fights and defends against the sources of surgical site infection (SSI). With CE Mark approval, CleanCision is now available to European hospital systems who are also facing higher than average surgical site infection rates in colorectal and other abdominal surgeries, nearly 27% according to a U.K. surveillance study.1 CleanCision received FDA 510(k) clearance in the United States in October, 2017.
CleanCision also received ISO 134385:2016 certification of its quality management system following completion of an audit by LNE/G-MED, the international body governing quality in medical device manufacturing. This certification demonstrates the company's focus on quality and demonstrates its ability to provide medical devices that consistently meet both customer and applicable regulatory requirements.
Infection control experts can see CleanCision at Booth 425 at the Association for Professionals in Infection Control and Epidemiology (APIC) Conference, June 13 ? 15, Minneapolis, MN.
"CE Mark and ISO certification are important milestones for CleanCision," said Jonathan Coe, president and CEO of Prescient Surgical. "Surgeons and infection control experts are an incredibly collaborative and global community that share best practices around surgical site infection prevention. Now, with both CE Mark Approval and FDA 510(k) Clearance, CleanCision can be readily integrated into emerging newer and more advanced infection control initiatives at major hospitals across Europe and the U.S."
Giving Surgeons More Control Over the Sources of Surgical Site Infection
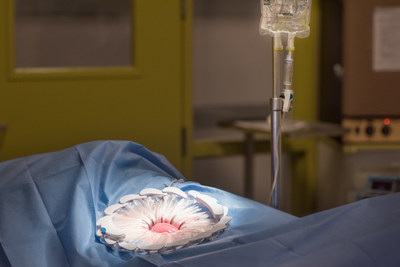
Unlike traditional intraoperative methods, which cannot continuously and consistently clear contamination from the surgical site, CleanCision combines wound protection and irrigation into an advanced, intuitive, and easy-to-use retraction system that actively, consistently, and continuously clears harmful bacteria that invades the incision during surgery.
Clinical data, recently published in The World Journal of Surgery, showed that CleanCision's combination of continuous irrigation and barrier protection was associated with a significant reduction in bacterial contamination.2
About Prescient Surgical
Based in San Carlos, Calif., Prescient Surgical, Inc. is a medical device innovator that makes advanced tools and technologies to fight and defend against the sources of surgical site infection.
For more information on CleanCisiontm and Prescient Surgical, follow us on Twitter @PrescientSurg and LinkedIn and visit http://www.prescientsurgical.com/
1. Tanner J, Khan D, Aplin C, Ball J, Thomas M, Bankart J. Post-discharge surveillance to identify colorectal surgical site infection rates and related costs. J Hosp Infect 2009;72:243-50.
2. Papaconstantinou, H.T., Ricciardi, R., Margolin, D.A. et al. (2018) 'A Novel Wound Retractor Combining Continuous Irrigation and Barrier Protection Reduces Incisional Contamination in Colorectal Surgery', World J Surg (Published online March 9, 2018) DOI: 10.1007/s00268-018-4568-z.
General contact: [email protected]

SOURCE Prescient Surgical
These press releases may also interest you
|
News published on and distributed by:



