Subjects: PDT, FDA
Roche launches the new cobas m 511 analyzer, signaling a new era in hematology testing
INDIANAPOLIS, March 8, 2018 /PRNewswire/ -- Roche (SIX: RO, ROG; OTCQX: RHHBY) announced today that its new hematology testing solution, the cobas m 511 integrated hematology analyzer, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
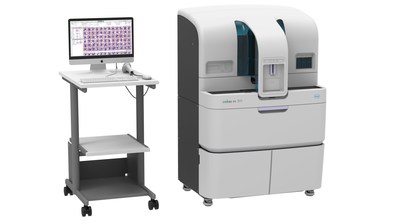
The cobas m 511 analyzer, featuring patented Bloodhound® technology, is designed to address the challenges of hematology testing by combining a cell counter, slide maker and stainer and a digital morphology analyzer into one integrated solution. Now, laboratories can report the complete blood count (CBC), white blood cell differential and reticulocyte results, and prepare, stain and analyze blood smears for abnormal results through a single, integrated process.
Traditional hematology testing, which is labor and time intensive, requires a larger blood sample that often needs to be processed using multiple methods to obtain a complete result. This challenge is further impacted by the fact that manual morphology may be difficult for laboratorians to interpret consistently, due to the varying qualities of stain and blood smears.
"The approval of the new cobas m 511 analyzer signals a new era of innovation in digital hematology testing," said Chief Medical Officer Dr. Alan Wright, Roche Diagnostics Corporation. "This integrated laboratory solution is a game-changer for patients and clinicians by offering faster access to abnormal results and potential accuracy gains through digitalization. One in 76 people in the U.S. are born with a blood disorder, making this aid in the diagnosis of blood diseases like anemia and leukemia a significant advancement."
Compared to current flow-based technologies, the cobas m 511 analyzer provides additional opportunities to improve the sensitivity and consistency of results by identifying, counting and categorizing white blood cells, red blood cells and platelets and then presenting the digital images of all these cell types. This enables medical technologists to concentrate their time on classifying abnormal cells within patient samples. Additional benefits of this automation and digitalization include reducing the need for resource-intensive microscope reviews and sample handling, easy collaboration for challenging cases through remote connectivity, and delivering quicker results, which ultimately aid patient diagnoses.
About the cobas m 511 and Bloodhound® technology
The cobas m 511 analyzer uses a unique approach to enter the new field of digital hematology through patented Bloodhound® technology for printing, staining and imaging. This technology uses only 30?L of blood to print a monolayer onto the slide, stains with an improved method for further analysis of the morphology and enables classification of cells displayed on a viewing station.
Contrary to the commonly used indirect methods in blood analysis today - primarily impedance and flow cytometry - the cobas m 511 images individual cells directly. Based on these direct images, the Bloodhound® technology counts, analyses morphology and then classifies cells in the viewing area to provide a standard CBC, five-part differential, and reticulocyte count. While hematologists will continue to have the option of microscopic review; the cobas m 511 provides cell-by-cell images that, in many cases, may eliminate this need.
About Roche
Roche is a global pioneer in pharmaceuticals and diagnostics focused on advancing science to improve people's lives. Roche is the world's largest biotech company, with truly differentiated medicines in oncology, immunology, infectious diseases, ophthalmology and diseases of the central nervous system. Roche is also the world leader in in vitro diagnostics and tissue-based cancer diagnostics, and a frontrunner in diabetes management. The combined strengths of pharmaceuticals and diagnostics under one roof have made Roche the leader in personalized healthcare?a strategy that aims to fit the right treatment to each patient in the best way possible.
Founded in 1896, Roche continues to search for better ways to prevent, diagnose and treat diseases and make a sustainable contribution to society. Twenty-nine medicines developed by Roche are included in the World Health Organization Model Lists of Essential Medicines, among them life-saving antibiotics, antimalarials and cancer medicines. Roche has been recognized as the Group Leader in sustainability within the Pharmaceuticals, Biotechnology & Life Sciences Industry nine years in a row by the Dow Jones Sustainability Indices (DJSI).
The Roche Group, headquartered in Basel, Switzerland, is active in over 100 countries and in 2017 employed more than 94,000 people worldwide. In 2017, Roche invested CHF 10.4 billion in R&D and posted sales of CHF 53.3 billion. Genentech, in the United States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical, Japan. For more information, please visit www.roche.com.
All trademarks used or mentioned in this release are protected by law.
For further information, please contact:
Christina Vysma ([email protected])
(317) 292-2920

SOURCE Roche
These press releases may also interest you
|
News published on and distributed by:



