Subjects: RCL, PSF
PharMEDium Services, LLC Expands Voluntary Nationwide Recall of Additional Lots of Compounded Sterile Products Within Expiry Due to Lack of Sterility Assurance
LAKE FOREST, Ill., Jan. 10, 2018 /PRNewswire/ -- PharMEDium Services, LLC (PharMEDium) is voluntarily expanding the recall issued on December 27, 2017 to include the below lots of sterile drug products to the hospital/user level due to a lack of assurance of sterility. Administration of a drug product intended to be sterile that is not sterile could result in serious infections that may be life-threatening. To date, PharMEDium has not received any reports of adverse events related to the products but is issuing this recall out of an abundance of caution following a commitment made during a recent inspection of the company's facility.
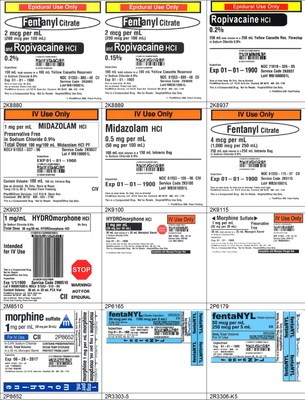
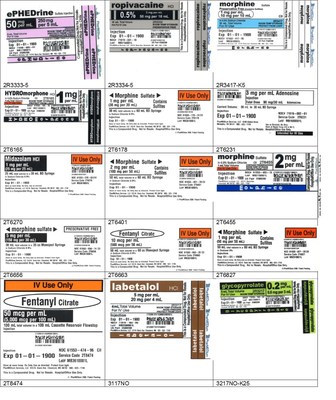
The recall is being expanded because PharMEDium conducted an extensive review of all commercially distributed product lots compounded in the Memphis location currently within their labeled expiration date in response to an FDA request regarding microbial program controls for the ISO5 environment, personnel glove sampling results, media fill results, sterility testing results, and endotoxin results following the most recent inspection. The original recall included a total of 55 lots of different products impacting 25,327 units. The expanded recall includes all lots within expiry compounded at our Memphis, TN facility. Finished product release testing for both sterility and endotoxin were acceptable for all lots. Although there were no defects identified in these products, as a conservative measure, this recall is being expanded.
The products can be identified by referring to the sample labels provided below. These products were distributed nationwide in the USA to hospitals/clinics. A complete list of all recalled products can be found on pharmedium.com.
PharMEDium Services is notifying customers of the voluntary recall by phone and email. Customers that have any of the affected medications that are being recalled should immediately quarantine the product, discontinue use and destroy per their hospital protocol. Customers with any of the affected medications can also reference PharMEDium Services website (pharmedium.com) for more information on the specific lot numbers affected and contact information.
Patients and healthcare providers with questions regarding this recall can contact PharMEDium Services Clinical Pharmacist at 1-800-523-7748, Monday through Friday, between 8am and 5pm Central Standard Time or via e-mail at [email protected].
Patients should contact their physician or healthcare provider if they have experienced any problems that may be related to the use of these products.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.


SOURCE PharMEDium Services, LLC
These press releases may also interest you
|
News published on and distributed by:



