Subject: FDA
EYE TECH CARE Receives CFDA Approval for its EyeOP1(R) Glaucoma Treatment product
SHANGHAI and LYON, France, Oct. 31, 2017 /PRNewswire/ -- Award-winning medical technology company EYE TECH CARE has received approval from the China Food and Drug Administration (CFDA) to begin marketing its EyeOP1® glaucoma treatment product in China.
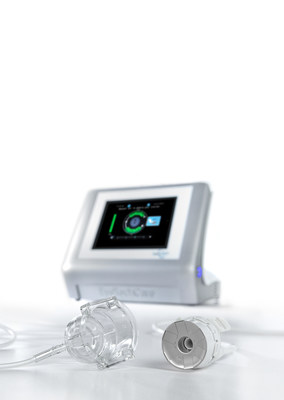
The first non-invasive medical device approved for the treatment of glaucoma in China, EyeOP1® treats glaucoma with an innovative, high-intensity focused ultrasound technology. Several clinical studies have been conducted, including in China, in order to prove the benefits of the technology, with 5,000 patients already treated.
"We are very happy to have reached the key milestone of CFDA approval," said EYE TECH CARE CEO Dr. Dietrich Wolf. "We are convinced that our technology will bring high value to the glaucoma market in China and provide new treatment options to patients. Along with our shareholders from China and Europe, we believe that this is a great example of cross-border cooperation between France and China, and the beginning of a promising journey for our company. We are now switching gears to prepare for commercial launch in China."
Glaucoma affects 120 million people globally and is the second most common cause of blindness after cataract. China has the largest population affected by glaucoma with 22 million patients, a number growing at an average rate of 7.5 percent per year according to Market Scope.
By the time they are diagnosed, many patients already have advanced glaucoma, which often requires an invasive surgical procedure that comes with associated risks. EyeOP1® provides a high-value alternative for patients suffering either from open-angle or angle-closure glaucoma, which has a particularly high incidence in China.
The key benefits to using high-intensity ultrasound technology include:
- Totally non-invasive approach minimizes risks associated with other traditional options
- A standardized and quick procedure that takes less than three minutes
- Short learning curve, high reproducibility, light clinical follow-up
Professor Sun Xinghuai, Chairman-designate of the Ophthalmology Branch of the Chinese Medical Association and Professor at Eye and ENT Hospital of Fudan University in Shanghai, said: "Ultrasound Cyclo Plasty (UCP) treatment with the EyeOP1® innovatively focuses ultrasound energy onto the ciliary body. The vast majority of patients have no complaint of ocular pain and have relatively minor side effects. For patients, UCP is a moderate and well-tolerated treatment."
Professor Ge Jian, Honorary Chairman of the Ophthalmology Branch of the Chinese Medical Association and Professor at the Zhongshan Ophthalmic Center of Sun Yat-sen University in Guanzhou, said: "Focused ultrasound technology provides glaucoma specialists with a new weapon that is non-invasive, safe and effective. Glaucoma specialists in China will welcome this technique, which can be combined with a variety of other weapons to develop appropriate, convenient, affordable and effective individualized treatment for patients."
About EyeOP1®
EyeOP1® is an innovative technology that uses high-intensity, focused ultrasound delivered by a miniaturized and computer-controlled eye probe to decrease the production of aqueous humor by partially coagulating the ciliary body, decreasing the intraocular pressure. It is the only computer-controlled glaucoma device with a treatment time of only 3 minutes.
About EYE TECH CARE
EYE TECH CARE is a French medical device company that has developed proprietary ultrasound technology to treat glaucoma. The company has received several awards for the design of its product, including the Frost & Sullivan Technology Innovation Award in 2016 and the MD&DI Silver Excellence Award in 2017.
For more information please visit www.eyetechcare.com.
Photo - https://mma.prnewswire.com/media/593786/Module_and_probe.jpg
These press releases may also interest you
|
News published on and distributed by:



