Subject: CON
Department of Veterans Affairs to Provide New Treatment Options for Veterans Suffering from Major Depressive Disorder
MORRISVILLE, N.C., Oct. 17, 2017 /PRNewswire/ -- Magstim, a pioneer in the study and development of Transcranial Magnetic Stimulation (TMS), has received a contract from the Veterans Administration to help treat Major Depressive Disorder (MDD) in Veterans who have failed to achieve satisfactory improvement from prior antidepressant medication.
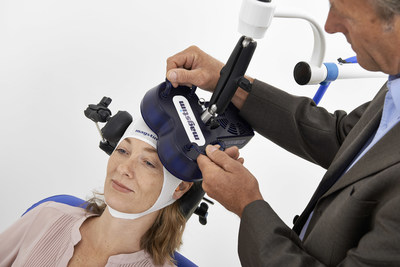
TMS is a non-invasive procedure that employs magnetic fields to stimulate nerve cells in the brain to reduce symptoms of depression. The device has been approved by the Food and Drug Administration (FDA) whose indication statement describes TMS as "an effective therapy for adults with MDD who have failed to achieve satisfactory improvement from prior antidepressant medication in the current episode."
The RAND Center for Military Health Policy Research says 300,000 (~20%) of the 1.7 million Veterans who served in Iraq and Afghanistan suffer from post-traumatic stress disorder or major depression. It has been estimated that 14% experience depression after deployment ? though the percentage is almost certainly higher because many Veterans don't seek care. In addition, active military service members are particularly susceptible. The same research indicates 19% have experienced traumatic brain injuries during combat which can also trigger depressive symptoms.
TMS is performed in a doctor's office or clinic with the patient seated in a comfortable chair while a "coil" is placed against the head. A series of repetitive, brief and highly-focused magnetic pulses are emitted to stimulate brain cells and, in depressed patients, restore brain activity to normal levels. Treatment sessions last less than 45 minutes and are typically performed five days per week, over a six-week period.
Magstim's TMS therapy is non-invasive and has very few side effects. Lothar Krinke, PhD, Group CEO at Magstim, says, "We are delighted to have this opportunity to employ Magstim TMS systems to increase patient access to treatment and improve health outcomes for Veterans. We are committed to working with the Department of Veterans Affairs as they move the field of psychiatric care forward."
Contact:
Alan Zachary
[email protected]
708/707-6834

SOURCE Magstim
These press releases may also interest you
|
News published on and distributed by:



