Subjects: SVY, TRI
NobleStitchtm EL Closure Data Presented At CSI UCSF Congress Compares Published RESPECT And CLOSURE PFO Data In New England Journal Of Medicine To NobleStitchtm
ROME, Oct. 9, 2017 /PRNewswire/ -- Prof. Dr. Achille Gaspardone, Director of Cardiology at Hospital of Sant'Eugenio (Rome, Italy) presented a comprehensive report on closure of PFO (Patent Foramen Ovale) and related Atrial Septal Defect utilizing the NobleStitchtm EL suture based closure system.
The data was collected from 10 centers throughout Italy and one center in Sweden from January 2016 through August 2017, and was gathered from more than 190 patients, from procedures performed by 14 interventional cardiologists. These patients were undergoing PFO closure for Stroke, Transient Ischemic Attack, Pulmonary Embolism, Decompression Illness and migraine with positive MR+. All patients were successfully treated with the NobleStitchtm EL closure system.
Dr. Gaspardone compared the NobleStitchtm EL data for closure rate, and adverse events (complications) using the same criteria listed in the recently published RESPECT and CLOSE clinical studies that were just published in the most recent New England Journal of Medicine. The NobleStitchtm EL demonstrated a superior closure rate to both the Gore Helix® and AGA Amplatzertm for closure with a zero residual shunt rate at 12 months follow-up. Additionally, the NobleStitchtm EL had no complications compared to the 4.2% complication rate found in the RESPECT trial and a 12.8% complication rate found in the CLOSE trial.
Dr. Gaspardone also presented a comparison to the previous PFO closure studies CLOSURE 1 and PC Trial and demonstrated a superior closure rate than found in the CLOSURE 1 study and equivalent closure rate to the PC Trial and more importantly the NobleStitchtm EL which had no complications compared to the CLOSURE 1 16.9% complication rate and the PC Trial 21% complication rate.
Dr. Gaspardone stated, "This initial registry included all patients treated and included the learning curve cases at each center. Unlike the afore mentioned studies using the umbrella based devices which were performed primarily by physicians with extensive experience implanting the umbrellas over many years, The NobleStitchtm EL Registry Data was collected with many users performing their very first cases. The NobleStitchtm EL is a simple, safe and effective way to close the septal defects without leaving behind a large metal prosthesis in the heart and without the risk of known complications of the umbrella implants which include, atrial fibrillation, migration, erosion, left ventricular thrombosis and nickel allergy." Dr. Gaspardone further stated, "Although we have experience with more than 300 cases with this technology we still continue to improve our techniques and outcomes with each new case, and I expect to see even greater increase in the positive data presented so far. Personally, if it were my family member or myself, as it is with all my patients, the NobleStitchtm EL suture would always be my first choice to close PFO."
Professor Anthony Nobles, Chairman, CEO and Chief Clinical Specialist of HeartStitch®, commented: " We were very pleased to have this data presented at such an important congress by one of the preeminent PFO experts in Europe. Dr. Gaspardone is known for his exceptional scientific clinical analysis and clinical technical skills in the cath lab. This data demonstrates that the NobleStitchtm EL can provide a safe and effective suture only alternative for patients who need their PFO closed without the concerns that many of these patients have expressed about having a large metal implant in their heart. We continue to expand the NobleStitchtm EL throughout Europe and Central Asia, and we believe our data will continue to demonstrate that patients treated with the NobleStitchtm EL can expect equal to or better closure results without many of the significant complications associated with the implantable metal umbrella devices."
About PFO Closure
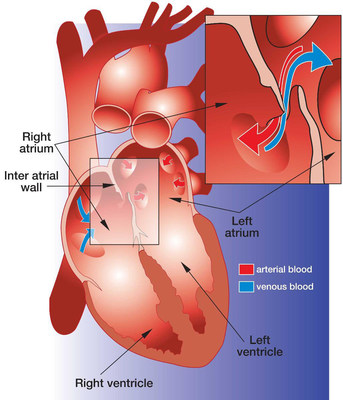
A PFO is a relatively common heart defect characterized by an unsealed tunnel between the right and left atria of the heart. This defect has been known to be present in anywhere between 27%-38% of people. However, in a number of cases, it is benign.
The PFO is formed as a trace of the fetal circulation. When the chambers of a human heart begin to develop, a tunnel is made between the right and left atria, allowing blood to flow directly from the venous circulation to the arterial circulation, circumventing the non-functioning fetal lungs. Following birth, the pressure differential between the right and left atria changes with newly operational blood flow to the fully functioning lungs. Because of this, the tunnel eventually closes completely within the first few months.
However, in some patients, the foramen ovale fails to seal and stays "patent". In patients with a Patent Foramen Ovale (PFO), the tunnel can reopen under elevated atrial pressure, such as coughing, or straining.
A key issue with PFO is that it gives a pathway for blood clots to pass directly to the arterial circulation without being filtered out by the capillary bed of the lungs. A PFO can also let deoxygenated blood and certain chemicals cross over to the arterial side. The presence of a PFO has been linked to a number of clinical issues, mainly strokes, migraines and chronic fatigue. Developments are being made to solidify the link between PFO and strokes or migraines, and to identify patients that would benefit from PFO closure.
About Nobles Medical Technology II
Nobles Medical Technology II, Inc. was founded by Prof. Anthony Nobles with the intent of leveraging its technologies in the PFO, ASD-closure, and cardiovascular-suturing marketplace. The company does business under the name of Nobles Medical II (NMT II). Initial efforts of the company have been focused in Europe on the innovative suture-based PFO closure system for closing the Patent Foramen Ovale (PFO), a tunnel between the right and left atria of the heart.
The NobleStitchtm is approved for PFO Closure and Cardiovascular suturing in the European Union.
The NobleStitchtm EL is FDA cleared for Vascular and Cardiovascular suturing in the United States. Further information including warnings and precautions can be found in the instructions for use.
NobleStitchtm EL is distributed worldwide by HeartStitch®, Inc. (HeartStitch® is a registered trademark of HeartStitch, Inc.).
NobleStitchtm EL for PFO closure
Covered by or for use under U.S. and international patents including one or more of U.S. Patent Nos. 5860990, 6117144, 6245079, 6551331, 6562052, 6733509, 7004952, 7090686, 7803167, 8197497, 8197510, 8246636, 8348962, 8372089, 8469975, 8496676, 8709020, and 9131938.
For more on Nobles Medical Technologies II visit http://www.noblesmed2.com.
For more information, please contact shareholder representatives:
Dru Dobbs
P. +1 714 427 6348
F. +1 714 427 6343
[email protected]

SOURCE Nobles Medical Technology II, Inc.
These press releases may also interest you
|
News published on and distributed by:



