Subjects: PDT, FDA
Interson's Latest FDA 510(k) Clearance Just Made The Future Of Ultrasound Healthcare More Accessible
PLEASANTON, Calif., June 13, 2017 /PRNewswire/ -- Interson announced today they have received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market their SiMPLi Series ultrasound array probes for medical applications. Interson has been the market leader in open-architecture USB ultrasound imaging products since 2007.
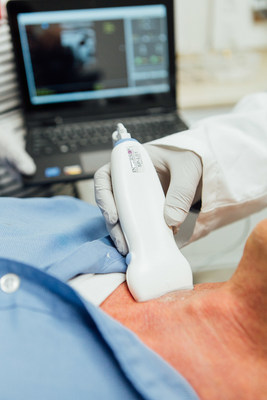
Interson's new linear and convex array probes enable medical professionals to use Windows tablets and laptops to display real-time ultrasound images at resolution and detail that were, until now, only available on high-end ultrasound systems. The new SiMPLi Series probes are lightweight, handheld and can easily be carried to any patient location. The SiMPLi Series probes provide such ease of use that now every medical professional can benefit. Simply connect the USB probe cable to a Windows computer to view real-time, high-resolution ultrasound images.
"These new array probes continue on our company vision to bring affordable, high-quality ultrasound imaging to every medical professional. SiMPLi Series is truly a portable, bedside ultrasound solution. We are proud to keep pushing the market forward on performance and practicality while also driving the cost of healthcare lower." Roman Solek, President & CEO
Interson's next-generation USB probes provide the image quality necessary for a wide variety of ultrasound diagnostic applications: (For image examples follow @simpliinterson on Twitter and @simpliultrasound on Facebook)
- Primary Care
- Emergency Medicine
- Abdominal & Vascular
- Musculoskeletal
- Telemedicine
Interson's open architecture solutions provide the capabilities and features that previously could only be addressed by more expensive, proprietary closed-architecture solutions. SiMPLi Series portable ultrasound imaging solutions can be quickly and easily brought to the patient, making point-of-care diagnostic medical imaging more timely and convenient. With a history of innovations recognized by the International Academy of Science, the new SiMPLi Series aims to follow by extending ultrasound diagnostic healthcare to the fingertips of all medical professionals.
Interson Corporation, an ISO certified, privately held corporation located in Silicon Valley, is a leading U.S. manufacturer of ultrasound imaging solutions. Ultrasound systems using Interson products have been installed in offices, hospitals, and clinics around the world since 1989. Interson ultrasound imaging probes are designed and manufactured in the U.S.A. and have FDA 510(k) clearance, a CE mark, Japan PAL NINSHO approval, and a medical device license from Health Canada.
Contact: Gregory DePaco Phone: 925-462-4948 Email: [email protected]
https://www.interson.com
SOURCE Interson
These press releases may also interest you
|
News published on and distributed by:



