Subject: FDA
New FDA-Cleared Smart Heart Monitor Keeps the Cardiologist a Heartbeat Away
SAN FRANCISCO, June 7, 2017 /PRNewswire/ -- Eko Devices ("Eko"), the leader in mobile acoustic cardiac monitoring tools, has received FDA clearance to market its latest innovation, DUO ? a combined digital stethoscope and electrocardiogram (ECG). The portable cardiac device was inspired by cardiologists' demand for more effective monitoring tools for heart disease ? the leading cause of death in the United States.
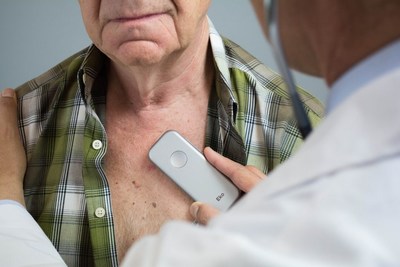
The marriage of ECG and digital stethoscope technology into a compact, handheld device offers unprecedented insight into cardiac function. Clinicians will use DUO as a cutting-edge screening tool or prescribe it to heart disease outpatients as the centerpiece of at-home health kits. The device wirelessly pairs with Eko's secure, HIPAA-compliant app, enabling remote monitoring and diagnosis by a clinician or specialist.
"Eko DUO's consumer-friendly design can help transform how clinicians monitor heart health in-person or virtually," said Dr. John Chorba, a Cardiologist and Assistant Professor at UC San Francisco. "We need powerful tools that heart failure patients can use to improve self-care and communicate troubling findings with an expert."
DUO represents a shift in the fight against heart disease, empowering patients to use familiar smart tools under the remote supervision of their physician to navigate complex medical conditions and, ultimately, reduce unnecessary hospital readmissions. Studies reveal that 25 percent of heart failure patients are readmitted to the hospital within 30 days and 50 percent are readmitted within six months. Heart failure costs the nation $30.7 billion annually.
"Cardiology programs are looking for ways to deliver hospital-quality healthcare at home," said Dr. Ami Bhatt, Director of Outpatient Cardiology and the Adult Congenital Heart Disease Program at Massachusetts General Hospital. "The ability to capture digital heart sounds and an ECG expands our portfolio of mechanisms to remotely monitor the heart, and brings diagnosis and opportunities for early intervention even further upstream. Robust toolkits for caring for patients in the community will hopefully lead to more appropriate healthcare utilization through continuous rather than episodic outpatient care."
Following its vision to improve cardiovascular care, Eko is also developing machine-learning algorithms that can be combined with DUO to automatically alert patients and their care teams of suspected decline in cardiac function.
For more information about DUO, please visit https://wp.ekodevices.com/duo-press-kit
Note: DUO is intended to be used by or with the prescription of a healthcare professional and is not available for over-the-counter purchase. This release also contains forward-looking statements concerning the future launch of Eko's clinical decision-support algorithms. Forward-looking statements are based on current expectations for future products and are not guarantees of future performance, development, or FDA clearance.
About Eko:
Eko is building a platform of non-invasive auscultation and cardiac screening devices, care coordination software, and point-of-care machine learning algorithms that enables more effective screening and management of cardiovascular diseases. Founded in 2013, the company is paving the way for an era of non-invasive, mobile, and intelligent cardiac care.
The company's first device, Eko CORE, is a smart stethoscope and accompanying platform now used by clinicians at over 700 hospitals & health systems around the globe and was recognized as a "Best Invention of the Year" by TIME Magazine. Eko's physician advisors include industry-leading cardiologists from Massachusetts General Hospital, UCSF, and the Mayo Clinic, and venture capital investors include the founders of Shazam, the founder of Splunk, Stanford University's StartX Fund, and DreamIT Ventures.
Media Contact: Tim Tassa
Email: [email protected]
Phone: 202-263-2580

SOURCE Eko Devices
These press releases may also interest you
|
News published on and distributed by:



